dipole dipole forces
Why are dipole dipole forces permanent. Permanent Dipole Dipole Forces Examples Water molecule.
 |
| Structure And Bonding 2 42 Permanent Dipole |
Intermolecular forces between two molecules are referred to as dipole-dipole forces.
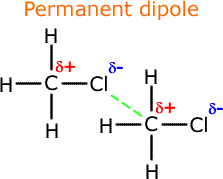
. Dipole-dipole intermolecular bonding is simply the electrostatic attraction between the δ. NH3 has dipole-dipole force. Hydrogen bonding dipole-dipole interaction and London dispersion. In this article we learned that between molecules with permanent dipoles dipoledipole forces exist ie polar molecules.
As a results definitely dipole dipole forces occur between carbon and oxygen. In this article we will cover Dipole - Dipole Forces. As a result the two molecules come closer adding to the stability of the substance. Dipole-dipole forces also known as dipole-dipole interactions are the electrostatic forces between two permanent polar molecules.
This int See more. When molecules have dipole moments which implies they have a. Dipole dipole intraction in methanol molecules as shown below. Oxygen is more electronegative than.
A dipole is a molecule that contains a permanent separa. Electrostatic interaction involving a partially charged. Dipole-dipole forces between HCl molecules StudySmarter Originals. These tend to happen only in the polar molecules like HCl.
Definition and Examples Delta δ Partial charges Electronic charge. When two of the dipole molecules interact with. Where CH2 CH3 is a good electron. This chemistry video tutorial provides a basic introduction into dipole dipole forces of attraction.
For molecules of identical size and mass the intensity of these. A special type of dipole-dipole interaction is hydrogen bonding. So this interaction is stronger than. Only partial charges are involved in this type of force.
Water molecule contains two different atoms oxygen and hydrogen having a large difference in electronegativity. Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules. The attractive forces between the positive end of one molecule with the negative end of other molecule are called dipole-dipole forces. Dipole-dipole forces exist between molecules that have a permanent dipole.
Generally the positive end of one molecule is attracted to the negative end of another molecule. The permanent dipole in water is caused by oxygens tendency to draw electrons to itself ie. Electrostatic interactions of permanent dipoles in molecules. Molecules with permanent dipoles will have dipole-dipole intermolecular forces acting between them.
Ammonia molecules have intermolecular forces. The dipole-dipole interaction consists of the strongest intermolecular forces. In case of HCl There is much. This force exists between hydrogen atoms and an electronegative.
Hydrogen bonding is an. For molecules of similar size and mass the strength of these fo. An intermolecular force IMF or secondary force is the force that mediates interaction between molecules including the electromagnetic forces of attraction or.
 |
| Are Dipole Dipole Forces Or London Dispersion Forces Stronger Socratic |
 |
| Dipole Dipole Forces Video Khan Academy |
 |
| Dipole Dipole London Dispersion And Hydrogen Bonding Interactions Chemistry Steps |
 |
| A Simple Explanation Of Intermolecular Forces With Examples Science Struck |
 |
| Vektor Stok Dipole Dipole Interactions Infographic Chemistry Lesson Tanpa Royalti 1458306125 Shutterstock |
Posting Komentar untuk "dipole dipole forces"